Introduction: Congenital neutropenia (CN) is characterized by chronic neutropenia due to a constitutional genetic defect.1 To date, these diseases have not been considered to be frequently associated with malignant solid tumors, unlike the risk of secondary myelodysplastic syndrome leukemia, which is well-known in CN.
Methods: The French Severe Chronic Neutropenia Registry (FSCNR) has prospectively enrolled CN patients since 1993. Solid tumors, identified during routine patient follow-up, were classified according to WHO criteria. We included localized lymphoma in the spectrum of malignant solid tumors. We calculated the incidence of malignant solid tumors in a cohort of CN patients.
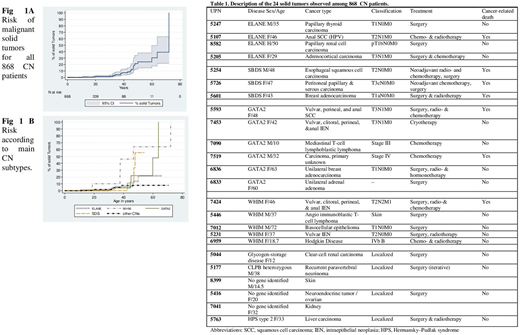
Results: Among 868 patients with various CN subtypes followed for a total of 16617 person-years, 24 patients who developed a malignant solid tumor were identified. Those cancers are described in Table 1, including the CN genetic anomaly. Cancers were almost always diagnosed in adulthood, with median age at diagnosis of 38.1 (range 10-72) years; only 3 cancers were diagnosed before age of 20 years. The cancer rate was 1.2% at 30 years of age, 7% at 40 years and 24% at 50 years (Fig. 1A). The risk-of-cancer percentages depended mainly on the associated genetic deficiency. Solid tumors were roughly distributed as follows: 33% among WHIM (CXCR4) patients, 5.3% among GATA2 patients, 2.7% among ELANE patients, 1.9 % among SBDS patients and 0.8% among for all other subtypes combined (Fig. 1B). Human papillomavirus (HPV) was the cause of cancer for 2/5 in WHIM patients and 2/6 in GATA2 patients. Three Lymphoma were identified, one in GATA2 patient and 2 in WHIM patients. Notably, our cohort's follow-up is skewed to the right, with less efficient monitoring of adults, with still limited long-term follow-up beyond 40 years. Therefore, we probably underestimated the solid-tumor risk in CN patients, as many patients, if alive, are no longer followed in hematology centers. Among 103 patients who underwent hematopoietic stem-cell transplantation (HSCT), 76 were long-term survivors. None of them developed solid tumors, which differs strikingly from the high malignancy risk associated with Fanconi anemia post-HSCT. Lastly, the FSCNR also includes and follows patients with idiopathic neutropenia. Among the 232 idiopathic neutropenia patients, followed for a total of 2866 person-years, no malignancy has been observed so far.
Conclusion: Our data lead us to advance that CN patients should be considered at risk of developing solid cancers, especially after the age of 30 years. This risk, at first glance, depended on the CN-associated genetic anomaly, with CXCR4 mutation, GATA2, SBDS and ELANE being the most frequent. HSCT was not associated with a higher risk and may, in contrast, be protective. These findings warrant confirmation but represent a compelling reason to prolong follow-up into adulthood of CN patients diagnosed during childhood. No indication was found of a specific high solid-tumor risk associated with idiopathic neutropenia.
Reference
Donadieu J, Beaupain B, Fenneteau O, Bellanne-Chantelot C. Congenital neutropenia in the era of genomics: classification, diagnosis, and natural history. Br.J.Haematol. 2017;179(4):557-574.
Acknowledgments: The French SCN registry is supported by grants from Amgen, Chugai, Prolong Pharma, X4 Pharma, Inserm, the Association 111 les Arts, the Association RMHE, the Association Sportive de Saint Quentin Fallavier. The authors thank the association IRIS and Mrs Grosjean and Mr Gonnot(ASSQF), the association Barth France for their support.
Hermine:Roche: Consultancy; Celgene BMS: Consultancy, Research Funding; AB Science: Consultancy, Current equity holder in publicly-traded company, Honoraria, Patents & Royalties, Research Funding; Alexion: Research Funding; Novartis: Research Funding. Blaise:Jazz Pharmaceuticals: Honoraria. Sicre de Fontbrune:Alexion Pharmaceuticals Inc.: Honoraria, Research Funding. cohen Beaussant:X4 Pharmaceuticals, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.